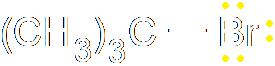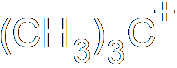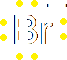Electrophiles
These "electron loving" species are always short of electrons. They have one of the following:
- An ion with a positive charge
- A polarised bond where one of the atoms has a δ+ charge on an atom. We say this sort of atom has an electron withdrawing group that has pulled electrons to one side of the molecule.
- A polarisable π bond
In a chemical reaction, an electrophile will always accept electrons. Examples include hydrogen halides, such as hydrogen chloride and hydrogen bromide. The bonds between the hydrogen and the halogen are very polarised, which is probably why the hydrogen halides ionise so easily.
|
Nucleophiles
These "nucleus loving" species always have a surplus of electrons. They have one of the following:
- a negative ion
- a pair of electrons in an orbital that are not used for bonding.
In a chemical reaction, an electrophile will always supply electrons. An example is methanol, which contains an oxygen atom with two p-orbitals. That's four electrons not used for bonding.
Another example is ethene. The double bond provides a cloud of electrons which can be used for bonding.
|
At A-level, you will need to spot which reactants are electrophiles and which are nucleophiles. It's normally a question of spotting the excess electrons in one reactant and the shortage in the other. Here are some examples:
- The addition of hydrogen bromide to ethene. This is a commonly used example, so you might well see it in your exam:
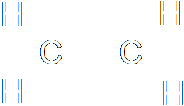 |
+ |
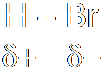 |
→ |
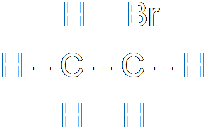 |
The pi electrons that form
the double bond in ethene
make it a nucleophile. |
|
The bond between the hydrogen
and the bromide is very polarised,
with the hydrogen atom having
a δ+ charge. |
|
- The substitution of methoxide ion for a chloride ion in chloroethane
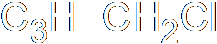
| + |
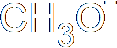 |
→ |
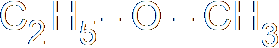 |
+ |
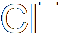 |
The carbon-chloride bond is polar,
with the C being δ+ and the
Cl being &delta-. The carbon is
therefore the electrophile. |
|
This ion is the
nucleophile as is negative. |
|
Exercise 1
In each of these reactions, please type the identity of the atom (e.g. carbon, nitrogen, hydrogen or whatever) that is the nucleophile and the identity of the atom that is the electrophile:
- CH3Br + OH- → CH3OH + Br-
Nucleophile:
Electrophile:
-
Nucleophile:
Electrophile:
-
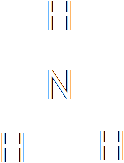 |
+ |
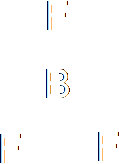 |
→ |
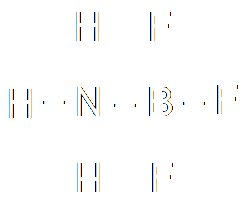 |
| Ammonia |
|
Boron
trifluoride |
|
For the life of
me, I can't find out
what this is called! |
Nucleophile:
Electrophile:
-
Nucleophile:
Electrophile:
- N3- + CH3Cl → CH3N3 + Cl-
Nucleophile:
Electrophile:
-
Nucleophile:
Electrophile:
Homolysis and Heterolysis
 These both concern the breaking of a covalent bond. You will recall, I'm sure, that a covalent bond involves the sharing of two electrons, one from each atom forming the bond, which end up orbiting both atoms in some fashion. In homolysis (a.k.a. homolytic fission), when the bond breaks, the two different atoms on either side of the bond each end up with one electron. The two halves of the molecule (assuming it breaks in half) form different free radicals, which aren't stable. Homolytic fission therefore occurs only as a stage in a chemical reaction, an intermediate step.
These both concern the breaking of a covalent bond. You will recall, I'm sure, that a covalent bond involves the sharing of two electrons, one from each atom forming the bond, which end up orbiting both atoms in some fashion. In homolysis (a.k.a. homolytic fission), when the bond breaks, the two different atoms on either side of the bond each end up with one electron. The two halves of the molecule (assuming it breaks in half) form different free radicals, which aren't stable. Homolytic fission therefore occurs only as a stage in a chemical reaction, an intermediate step.
In heterolysis (or heterolytic fission), both the electrons that form the bond pass to one of the atoms, the one that is more electronegative. Since one atom is gaining an electron and the other losing one, two ions must be formed, one negative and one positive. Here's an example, where a carbon-bromine bond breaks to form a positive ion on the carbon and a negative bromide ion. Of course, you don't get stable carbon ions in nature, so this is also nothing more than intermediate step in a larger reaction.
The prefixes homo- (same) and hetero- (different) should help you remember which is which. The bonds have a certain energy associated with them, and the energy needed to crack the bond is known as either the homolytic bond dissociation energy or the heterolytic bond dissociation energy, depending on how the bond breaks.
At A-level, you will need to be able to spot which reactions involve heterolytic fission and which involve homolytic fission. It's a question of knowing where the electrons must end up in order for it all to make sense:
Take the reaction that you saw above, in which a chloride on a carbon is replaced by a N3:
N3- + CH3Cl → CH3N3 + Cl-
The splitting of the bond must be heterolytic, since a chloride ion is produced. The chloride ion grabs both the electrons forming the bond from the carbon. The addition of hydrogen bromide to ethene is an example of homolytic fission. The first step of the reaction is that the pi-bond of the carbon-carbon bond breaks. Consider the two possibilities for the splitting of that bond. It could be homolytic or heterolytic:
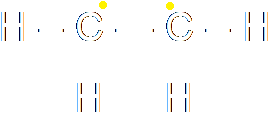 |
or |
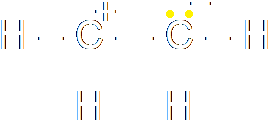 |
? |
The question is, which of these would happen? In this case, the molecule is perfectly symmetrical across the double bond. Neither carbon is more electronegative than the other, so there is no reason why the split would be heterolytic. This is an example of homolytic fission. The bond in the hydrogen bromide also breaks homolytically, so that the hydrogen radical ends up with one electron and the bromide radical ends up with the other. Then the hydrogen bonds to one carbon and the bromide to the other.

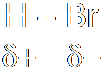
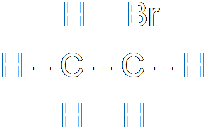
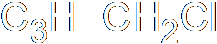
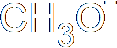
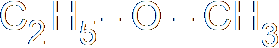
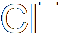
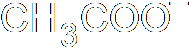

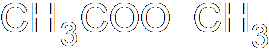
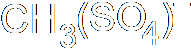

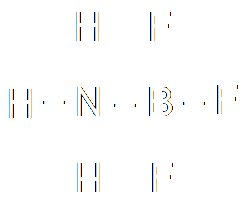

 These both concern the breaking of a covalent bond. You will recall, I'm sure, that a covalent bond involves the sharing of two electrons, one from each atom forming the bond, which end up orbiting both atoms in some fashion. In homolysis (a.k.a. homolytic fission), when the bond breaks, the two different atoms on either side of the bond each end up with one electron. The two halves of the molecule (assuming it breaks in half) form different free radicals, which aren't stable. Homolytic fission therefore occurs only as a stage in a chemical reaction, an intermediate step.
These both concern the breaking of a covalent bond. You will recall, I'm sure, that a covalent bond involves the sharing of two electrons, one from each atom forming the bond, which end up orbiting both atoms in some fashion. In homolysis (a.k.a. homolytic fission), when the bond breaks, the two different atoms on either side of the bond each end up with one electron. The two halves of the molecule (assuming it breaks in half) form different free radicals, which aren't stable. Homolytic fission therefore occurs only as a stage in a chemical reaction, an intermediate step.