The Reactivity (Electrochemical) SeriesA salt is a chemical containing a metal ion and a negative ion bonded together. The metal ions might consist of copper, sodium or zinc etc. The negative ions could be sulphate, chloride or oxide etc. Some metals are stronger than others. If a strong metal is mixed with a salt from a weaker metal, the strong metal grabs the negative ion from the weaker one. Here is an example:
is stronger than , so if is heated with , it reacts to give and .
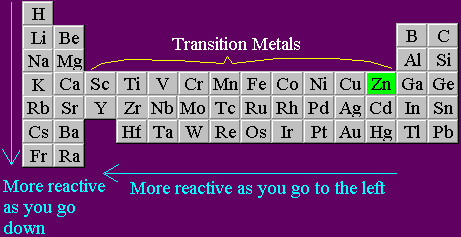
An element is more reactive if it is further to the left of the periodic table or further down. Transition metals mess up the pattern as they have special D electrons. Click on the names in the reactivity series above to see where the element concerned is in the periodic table. Electrons orbitting all atoms (whether they are metal atoms or not) are arranged into different "shells", at different distances from the nucleus. Metal atoms have only a few electrons in their outermost electron shells, and generally react by losing those spare electrons until the ones they are left with are just enough to complete full electron shells. 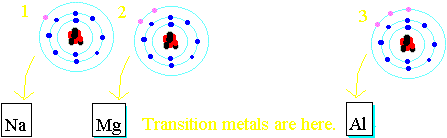 The reactivity of the metals decreases as you move right across the table because the atoms have more electrons that they need to lose. Energy is needed to strip those negative electrons away from the positive nucleus - since they are opposite in charge, they are attracted to each other - and the more electrons a metal atom has in its outer shell, the harder it is to get that atom to react. Sodium reacts by losing a single electron. Magnesium reacts by losing two electrons. Aluminium reacts by losing three electrons. Losing each electron requires some energy to be put in, so it is easier for sodium to react than magnesium, and easier for magnesium to react than aluminium.
This means that the nucleus exerts less of an electrostatic pull on the outer electron. The further down a group an atom is, the larger the atom is, and the more reactive the element is. Atoms of sodium (Na in the table) are larger, and so more reactive, than atoms of lithium (Li in the table). Similarly, atoms of potassium (K in the table) are larger, and so more reactive, than atoms of sodium. It follows that elements below potassium in the table should be more reactive than potassium is. Experiments show that Rubidium is indeed more reactive than potassium and Caesium is even more reactive than Rubidium.
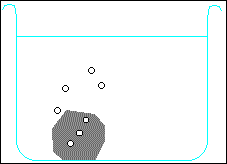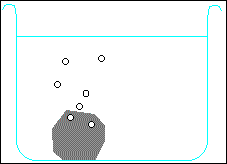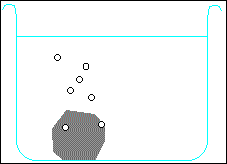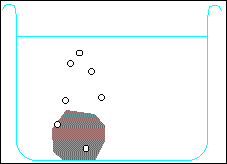
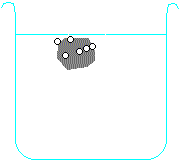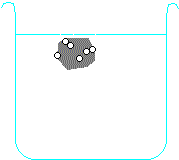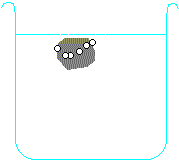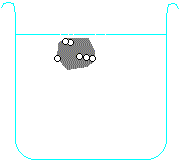
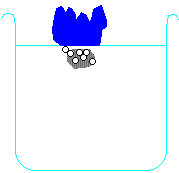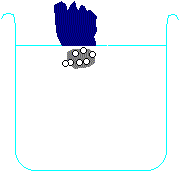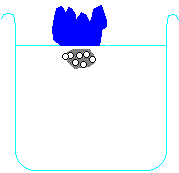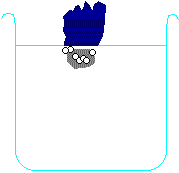
Metals higher than hydrogen in the table will also react with water, again producing hydrogen gas. Metals from calcium upwards will react spontaneously with water, but metals lower than that (e.g. magnesium or zinc) have to be heated with water in order to get them to react:
Potassium + water → potassium hydroxide + hydrogen
2 K + H2O → 2 KOH + H2 Calcium + water → calcium hydroxide + hydrogen Ca + H2O → Ca(OH)2 + H2 Magnesium + water → magnesium oxide + hydrogen Mg + H2O → MgO + H2 Zinc + water → zinc oxide + hydrogen Zn + H2O → ZnO + H2 Carbon is also often included in the reactivity series, and I have included it in the series above. Although it doesn't form salts (for instance, you never get carbon sulphate or carbon nitrate), it grabs oxygen atoms from oxides of unreactive metals e.g. copper or iron. Click on one of the elements in the reactivity series above to see if its oxide reacts with carbon. 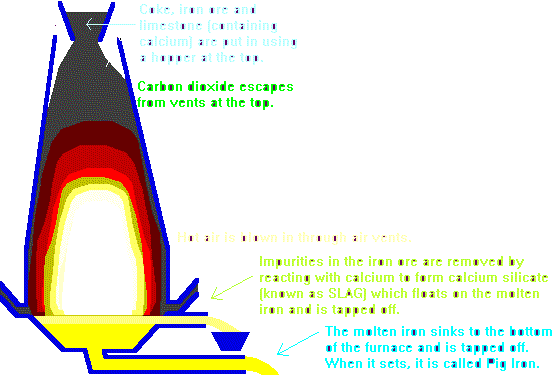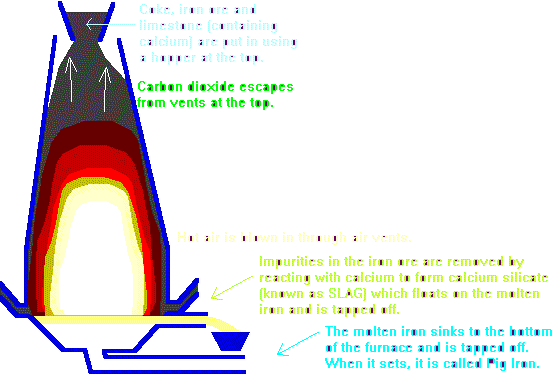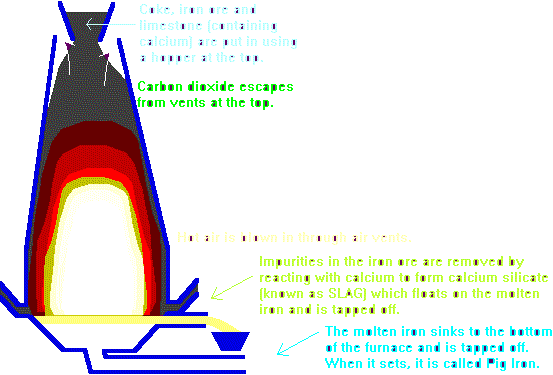
The Blast FurnaceCarbon, in the form of coke, is used to extract iron from iron ore (a mixture of iron oxides) by heating them together in a Blast Furnace. The Blast Furnace can be used to extract any metal lower than carbon in the reactivity series (e.g. lead or copper). Metals higher in the reactivity series (e.g. sodium or potassium) have to be extracted using Electrolysis. Although three oxides of iron exist in nature (FeO, Fe2O3 and the one forming the primary constituent of magnetite, Fe3O4), it is mainly Fe2O3 (Iron III oxide) that is used in the blast furnace. The reactions taking place in the blast furnace are actually quite complicated. In the highest section, carbon reacts with the limited amount of oxygen available to form carbon monoxide:
2 C (s) + O2 (g) → 2 CO (g)
Lower down in the furnace, the carbon monoxide reacts with the iron (III) oxide to produce carbon dioxide and molten iron:
3 CO (g) + Fe2O3 (s) → 2 Fe (l) + 3 CO2 (g)
Limestone (calcium carbonate, CaCO3) is added to the coke and iron ore to remove an impurity called silica (silicon dioxide, SiO2) that is found in the iron ore. If silica is allowed to remain in the iron after smelting, the resulting metal is brittle. In the intense heat, the limestone quickly breaks down to form calcium oxide ("quick lime"): CaCO3 (s) → CaO (s) + CO2 (g)
The calcium oxide is a base oxide, i.e. it would react readily with acids. Since silica is a weakly acidic oxide, it reacts with the calcium oxide to form calcium silicate: CaO (s) + SiO2 (s) → CaSiO3 (l)
The calcium silicate, an impurity called slag, falls to the bottom of the furnace where it forms a layer of sludge on top of the molten iron and is removed. | ||||||||||||||||||||||||||||||||||
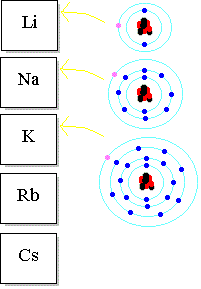 The reactivity of metals increases as you go down the group because the outer electrons (which are lost when the metal reacts) are further from the nucleus.
The reactivity of metals increases as you go down the group because the outer electrons (which are lost when the metal reacts) are further from the nucleus.
 Back to the main menu
Back to the main menu